THRUST AREA: Cellular Engineering
Project I: Supramolecular Aptamer Assemblies for Modulating Cellular Response
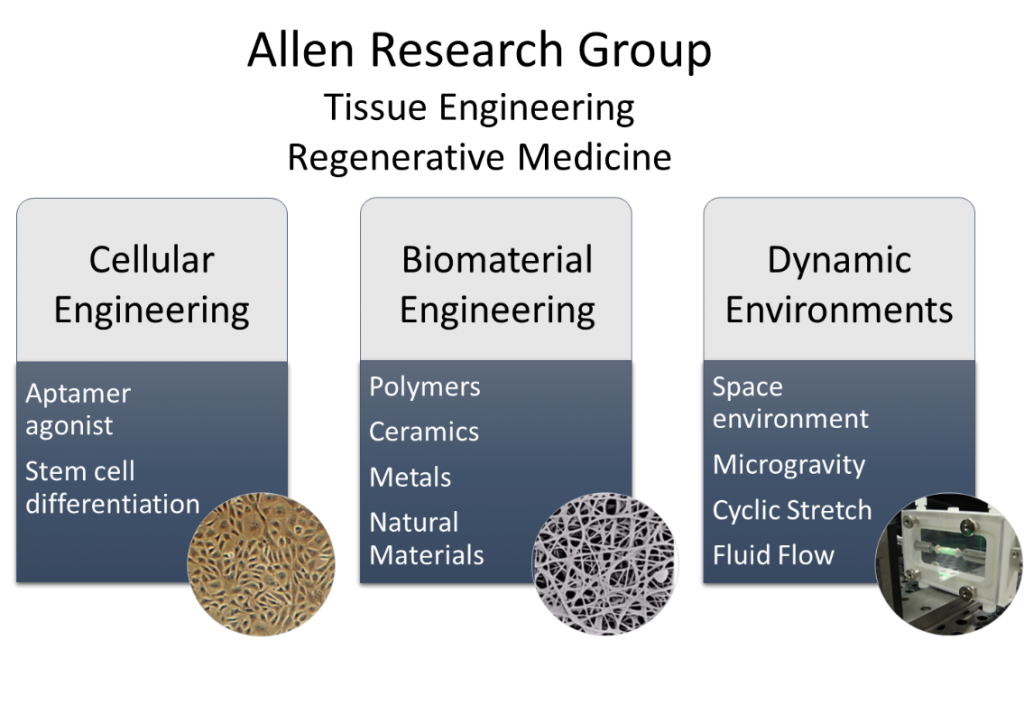
Schematic representation of the proposed method of action of the divalent aptamer assembly to target and activate through dimerization, membrane-bound vascular endothelial growth factor receptor-2 (VEGFR2)In the Allen Research Group, we focus on designing novel subcellular biopolymeric cues to specifically target and control receptor-mediated cell processes. We have previously developed a divalent aptamer assembly that specifically acts as an agonist causing receptor activation and of Vascular Endothelial Growth factor receptor 2 (VEGFR2) in endothelial cell systems. Currently, we are also interested in designing and developing supramolecular nucleic acid based aptamer assemblies that can trigger and activate downstream signaling pathways to promote cellular processes such as repair, differentiation, and growth for tissue engineering and regeneration. |
Project II: Controlling Stem Cell DifferentiationOur labs work to control stem cell differentiation is motivated by the need for functional cells for tissue engineering. One of our approaches involves the use of all-trans retinoic acid (ATRA), a natural molecule made in vivo from Vitamin A, that plays and integral role in many cellular processes. Particularly, research has shown ATRA has an effect on the direction of cell fate for multipotent cells. In the Allen Research Group, we are exploiting the differentiation capabilities of ATRA to differentiate adipose-derived stem cells (ADSCs) into a keratinocyte-like cellular phenotype. The goal of this work is to explore the utilization of biologically active molecules to differentiate stem cells and promote regeneration of dermal tissue. |
THRUST AREA: Biomaterial Engineering
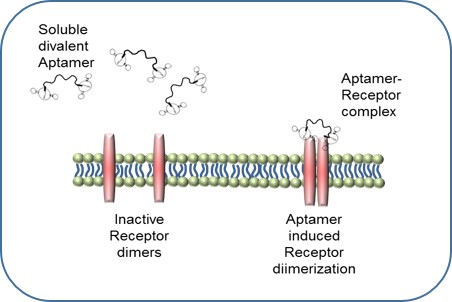
Project I: Small Diameter Vascular Grafts
|
Project II: Nanocomposite Scaffolds for Bone Tissue Regeneration
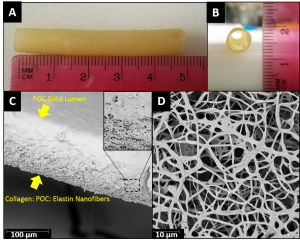
Digital image (a), scanning electron micrographs showing the structure of (b) and attachment of pre-osteoblast cells on POC/TCP/CNP nanocomposites.Polymeric nanocomposite scaffolds are utilized as synthetic alternatives to tissue grafts. The Allen Research Group is currently investigating unique biodegradable elastomeric composites with the ability to scavenge free radicals from the surrounding cellular environment. This construct is intended to reduce oxidative stress caused by free radicals which can lead to cellular damage and death. The goal of the project is to promote and increase the rate of bone regeneration. |
Project III: Electrospun Scaffolds for Wound Healing
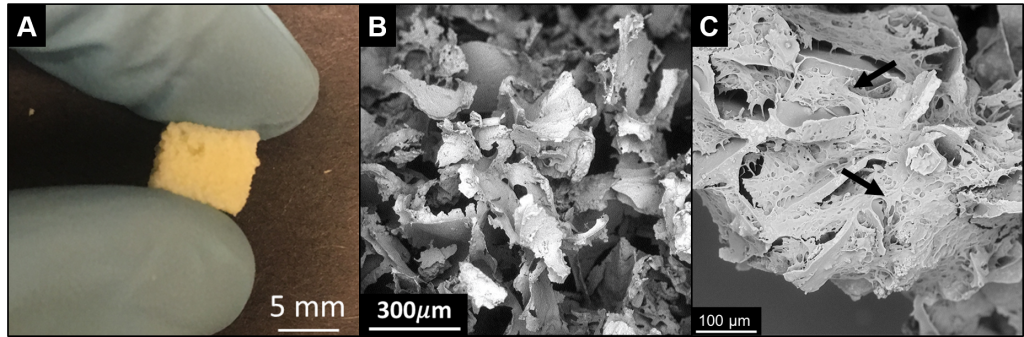
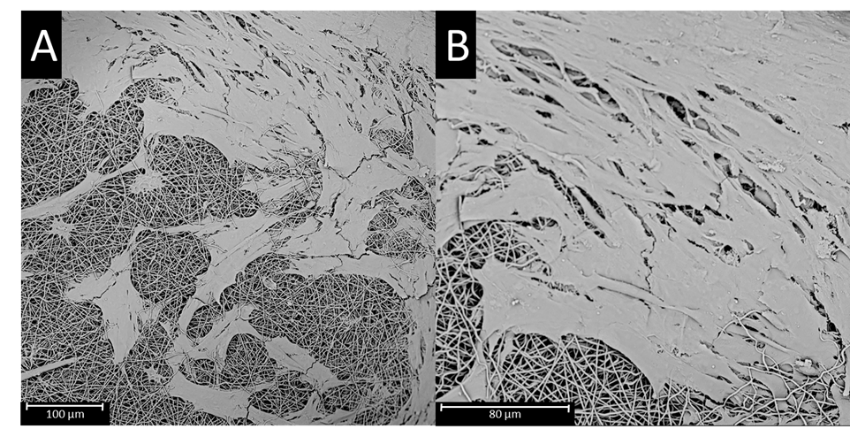
Representative SEM images of D551 cells on 50:50 POC:PAA scaffolds; 500x (a) and 1000x (b).Biologically inert dressings are currently the standard for wound dressing technology. In the Allen Research Group, we are using biodegradable polymers to fabricate wound dressings that prevent bacterial infection and promote tissue regeneration. Exploiting inherent chemical character of polymers and functionalizing with biologically active molecules we are fabrication bioactive wound dressings. |
THRUST AREA: Dynamic Environments
Project I: Vascular Repair Mechanisms in Altered Environmental Conditions
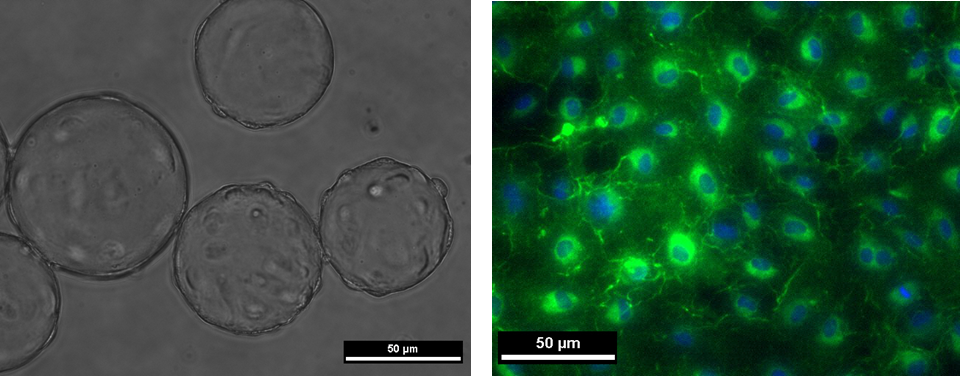
Microscopic Image of human stem cells attached and growing on carrier beads (a) Staining of transformed human stem cells showing evidence of the presence of characteristic mature cell proteins (b).The Allen Research Group also works on understanding vascular repair mechanisms in the body under altered conditions such as microgravity. We are specifically interested in studying vascular cell damage and associated cardiovascular complications in the space environment by exposing vascular cells to space flight or conditions simulating spaceflight. The goal of this work is to gain insight that will contribute to a greater understanding of cardiovascular deconditioning. We have previously studied the effect of simulated microgravity on the differentiation of blood derived circulating stem cells and their downstream functionality in altered environmental conditions. We are currently working on further expanding this pilot study, to assess changes in transcriptomics of vascular cell types in space compared to those in a ground-based study. |